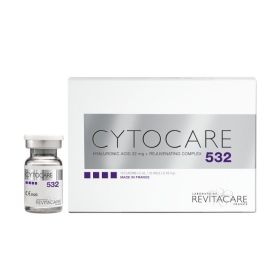
CYTOCARE 532 (10x5ml) REVITACARE
CYTOCARE 532, complex combined CT50 rejuvenating formula and hyaluronic acid 32 mg. REDUCTION Signs of age. Reduces wrinkles. Correct aging process of the skin. Restores tone and elasticity. High Hydration improves shine.

Worldwide Shipping via DHL

Genuine Products

100% Secure Checkout
CYTOCARE 532 (10x5ml) REVITACARE
For medical use only. Sterile single use medical devices class III.
DESCRIPTION
CYTOCARE BY REVITACARE is a range of resorbable implant composed of Hyaluronic acid + Rejuvenating complex, injectable by micro-injections into superficial dermis of the face to treat : fine lines and wrinkles, dehydrated skin and lack of density and radiance of the skin. Complex combined CT50 rejuvenating formula and hyaluronic acid 32 mg.
INDICATION
- PREVENTION Wrinkle reduction.
- Intense re-densification and dermal regeneration.
- Warns against premature aging.
- Delays the appearance of wrinkles.
- High Hydration, glow and radiance.
ACTIVE INGREDIENTS
Hyaluronic acid, microelement and multivitamin complex, all 20 essential amino acids, thymidine, putrescine, lipoic acid.
RESULTS
- Prevention of photoaging.
- Stress-induced tired skin appearance.
- As preparation for chemical peels, or laser skin resurfacing.
- Need for instant short-term improvement in appearance.
INJECTION AREA
Middle dermis or superficial dermis of the face or the neck. *Do not inject into eyes circles, glabella wrinkles & lips.
CHARACTERISTICS
- 5 vials x 10ml.
- Sterilization: aseptic filtration.
- Pyrogen free.
- Ready to use.
- Conservation condition: 2°C to 30°C.
- Close to neutral pH.
- Iso-osmolar solution.
INJECTIONS DEPTH
Middle dermis or superficial dermis of the face or the neck.
INGREDIENTS
Ammonium Molybdate, Ammonium Metavanadate, Calcium Chloride, Iron Sulfate, Potassium Chloride,Copper Sulfate, Magnesium Chloride, Manganese Sulfate, Sodium Acetate, Sodium Hydrogen Carbonate, Sodium Chloride, Sodium Hydrogen Phosphate, Sodium Metasilicate, Sodium Selenite, Nickel Chloride, Tin Chloride, Zinc Sulfate, Alanine, Arginine, Asparagine, Aspartic Acid, Cysteine, Glutamine, Glutamic Acid, Glycine, Histidine, Isoleucine, Leucine, Lysine, Methionine, Phenylalanine, Proline, Serine, Threonine, Tryptophan, Tyrosine, Valine, Adenine (Vit.B4), Biotin (Vit.B8), Calcium Pantothenate(Vit.B5), Choline Chloride, Folic Acid (Vit.B9), Inositol (Vit.B7), Nicotinamide (Vit.B3), Pyridoxine (Vit.B6), Riboflavin (Vit.B2), Thiamine (Vit.B1), Vitamin B12, Deoxythymidine, Glucose, Putrescine, Sodium Pyruvate, Lipoic Acid.